- Calculating Atomic Mass Examples
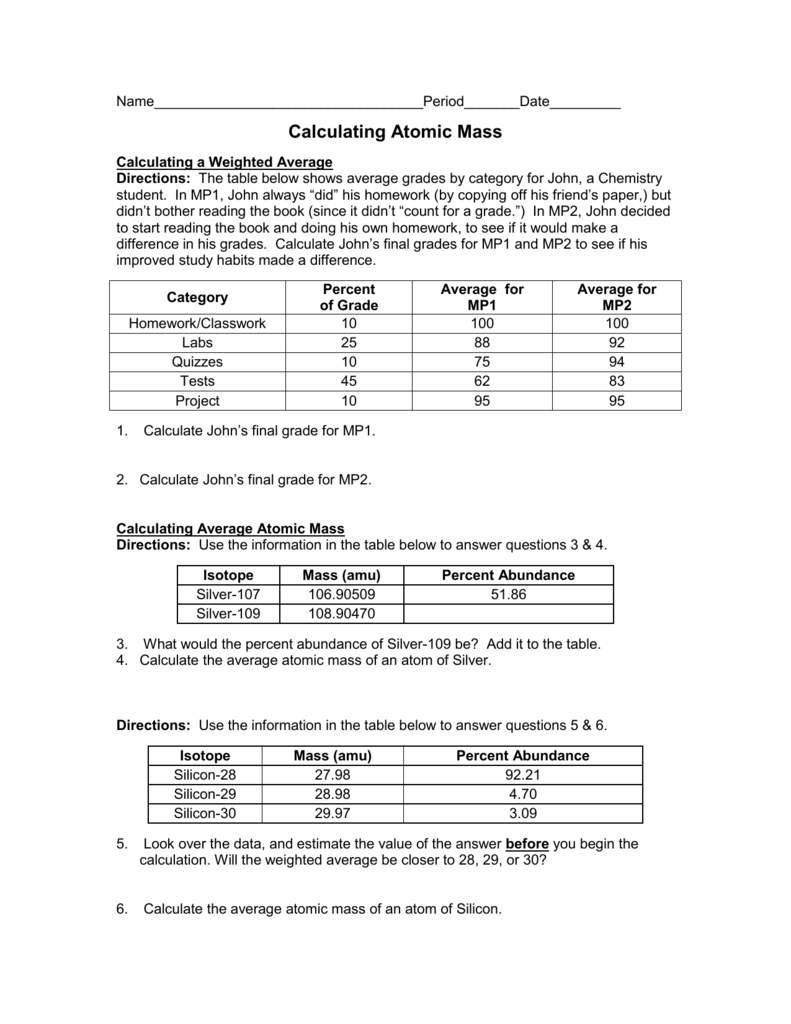
- Calculating Atomic Mass Worksheet
- Atomic Mass Formula
- Periodic Table With Atomic Mass
- Calculating Atomic Mass Abundance
How do you find the average atomic mass?
Calculating Atomic Mass Examples
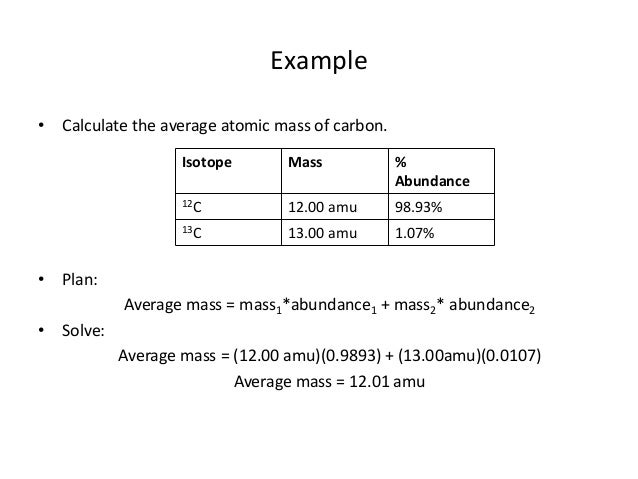
- Atomic mass is measured in atomic mass units (amu), where one amu is roughly equivalent to the mass of a single proton or neutron. The isotopes of an element do not occur in equal percentages in nature, so a weighted average must be taken in order to achieve the atomic mass of the element.
- Average atomic masses listed by IUPAC are based on a study of experimental results. Bromine has two isotopes, 79 Br and 81 Br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69% and 49.31%) were determined in earlier experiments. Calculate the average atomic mass of Br based on these experiments.
Atomic Mass calculator uses atomicmass = Number of Protons+Number of Neutrons+Number of Electrons to calculate the Atomic Mass, Atomic mass is the total of the masses of the electrons, neutrons, and protons in an atom. Atomic Mass and is denoted by M symbol. How to calculate Atomic Mass using this online calculator? Calculate Atomic Mass Atomic massis the sum of all the protons, neutrons, and electrons in a single atom or molecule. The mass of an electron is so small, it is considered negligible and not included in the calculation. Atomic mass can be defined as the total mass of one atom of any given element. The unit of atomic mass is called the unified atomic mass unit (denoted by ‘u’). Most of the atomic mass of a substance is made up of protons and neutrons. Therefore, it is almost equal to its mass number.
Calculating Atomic Mass Worksheet

1 Answer
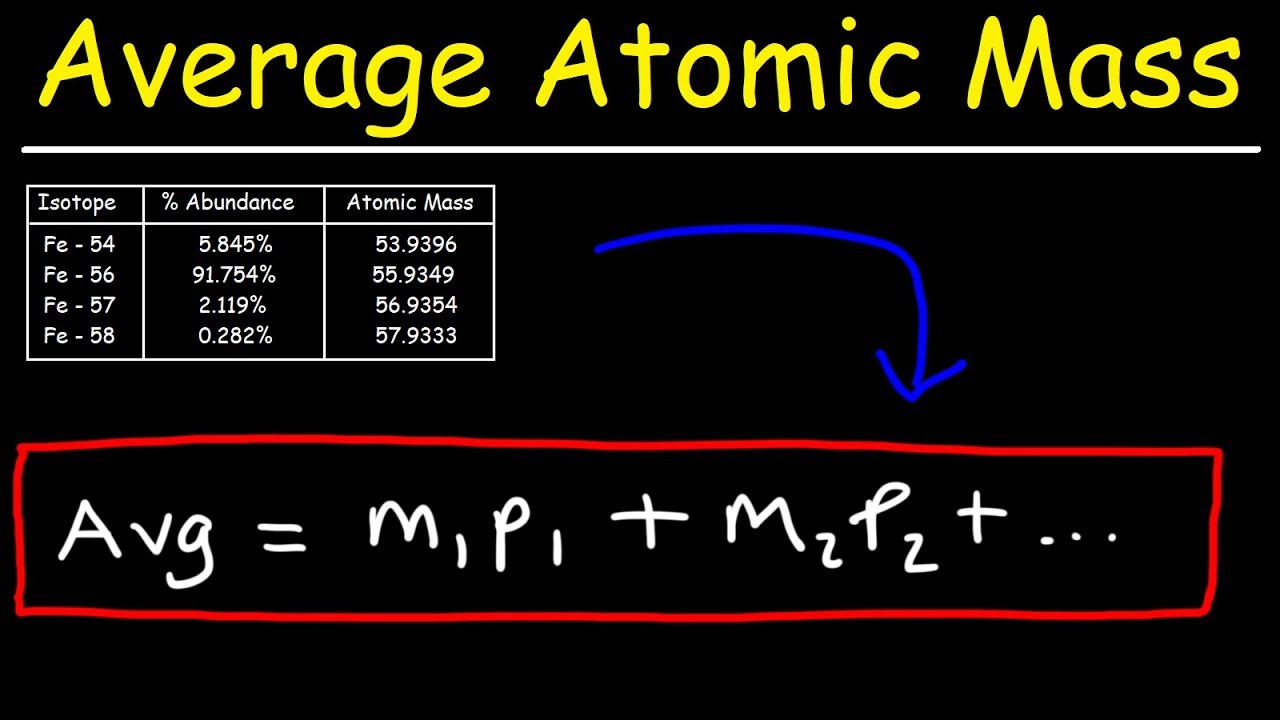
When calculating the average atomic mass of an element you should be given the isotope and the percent abundance. For example (X is a made up element).
Atomic Mass Formula
Note: amu stands for atomic mass unit
Given that information, you multiply the amu by the percent abundance
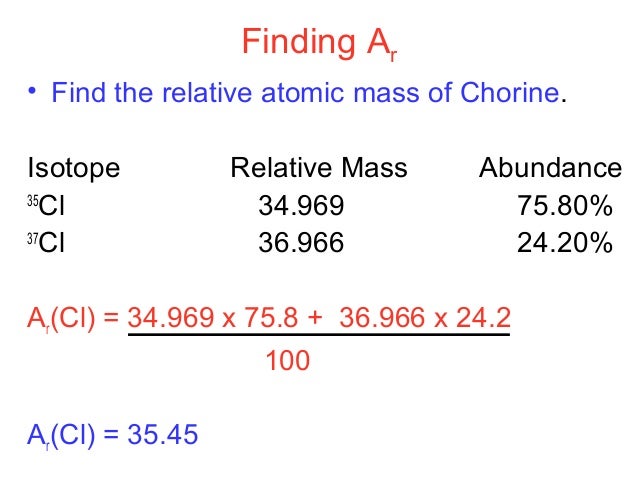
Then you add the two figures together to get your average atomic mass.
Periodic Table With Atomic Mass
Calculating Atomic Mass Abundance
Related questions

Comments are closed.